Understanding Oxidative Stress and Electron Transfer Imbalance
Written on
Chapter 1: The Role of Electron Transfer in Cellular Function
Electron transfer is vital for energy production in our cells; however, an imbalance can hinder our overall well-being.
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: Understanding Electrons and Their Transfers
Electrons are fundamental particles that facilitate the flow of electric currents, which is how we obtain electricity from outlets. They also play a crucial role in chemical reactions, where they can be transferred between molecules, altering their chemical structures and properties. This process is central to the concepts of oxidation and reduction.
When Chemical A extracts electrons from Chemical B, Chemical B undergoes oxidation (losing electrons), while Chemical A acts as a pro-oxidizing agent and is reduced. If Chemical B remains stable upon oxidation, it serves as an effective antioxidant. Conversely, if it becomes unstable, it turns into a reactive oxygen species (ROS) that must oxidize another molecule to regain stability. The cycle of these ROS reactions can produce even more unstable species, commonly referred to as "free radicals," which are frequently discussed across various health platforms.
Subsection 1.1.1: The Importance of Redox Potential

Redox potential plays a crucial role in determining the likelihood of ROS propagation. Different pairings of molecules yield varying redox potentials, as illustrated in the table below:
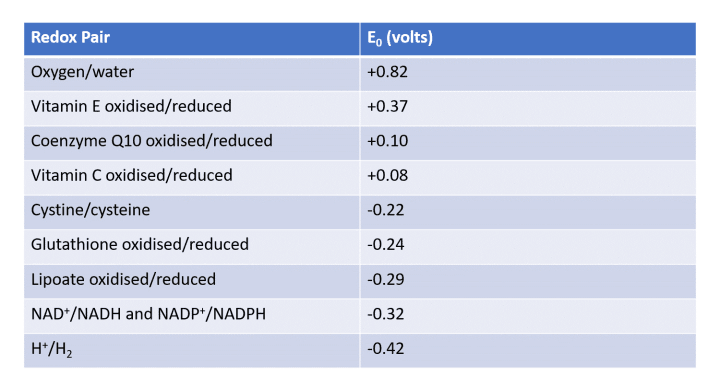
For instance, the redox potential of oxygen/water is +0.82 V, while that of oxidized/reduced glutathione is -0.24 V. This indicates that oxygen acts as the oxidizing agent, whereas water serves as its reducing counterpart. The higher redox potential of the oxygen-water pair compared to glutathione suggests that oxygen can oxidize reduced glutathione into oxidized glutathione, converting itself to water in the process.
If one were to analyze the interactions between reduced Vitamin C and oxidized Vitamin E, it becomes evident that Vitamin C can regenerate Vitamin E while itself becoming oxidized, underscoring the significance of understanding redox potential.
Section 1.2: The Regulation of Redox Activity
Redox activity within our bodies is typically well-regulated. Mitochondria produce a significant amount of ROS, but their activity is balanced by glutathione antioxidants generated through the nrf2 transcriptional pathway. Additionally, Coenzyme Q10 (CoQ10) plays a critical role in facilitating electron transport during energy generation. However, as we age, CoQ10 production decreases, particularly in individuals taking statins, which can further diminish its levels.
Chapter 2: The Implications of Oxidative Stress
This video titled "Oxygen, Stress, and Antioxidants" by Ron Mittler, PhD, explains the relationship between oxygen, stress, and the role of antioxidants in our health.
As ROS levels rise, an imbalance develops between these species and our antioxidant reserves, leading to oxidative stress. This phenomenon can significantly alter the structure and function of proteins, lipids, and DNA, ultimately affecting our biological systems.
The second video, "What is Oxidative Stress, Free Radicals & Antioxidants" by Katie Rose, delves deeper into oxidative stress and its implications for health.
Oxidative stress, while seemingly a chemical issue, reveals much about the underlying changes in our biological functions. When cells are exposed to biochemical stressors, their ability to function optimally is compromised, often leading to detrimental outcomes.
Maintaining a healthy balance of electron transfers is essential. While increasing antioxidant intake can be beneficial, it's important to note that many antioxidants can only neutralize ROS once. Unlike these, glutathione can be consistently regenerated within cells, highlighting the importance of internal antioxidant mechanisms.
As ROS accumulate, unneutralized molecules can target various biological structures, leading to potential health issues, including cancer from the consumption of charred foods, which can convert into harmful ROS.
In conclusion, the delicate balance of redox reactions is crucial for sustaining health, yet it often goes unnoticed until health problems arise.