The Explosive Discoveries of Potassium and Sodium in Chemistry
Written on
Chapter 1: Introduction to Electrochemistry
Many people might share a similar sentiment when they hear "chemistry"—a cringe, perhaps a flashback to an underwhelming academic experience. However, this series aims to shift that perspective, making chemistry accessible and enjoyable for everyone.
Historically, the analysis of substances often relied on combustion. But in the early 1800s, the advent of the "voltaic pile" introduced a new method of investigation that rivaled burning. Contrary to what the name may suggest, a voltaic pile is actually an early form of a battery. This device consisted of alternating layers of copper and zinc discs, separated by cloth soaked in saltwater.
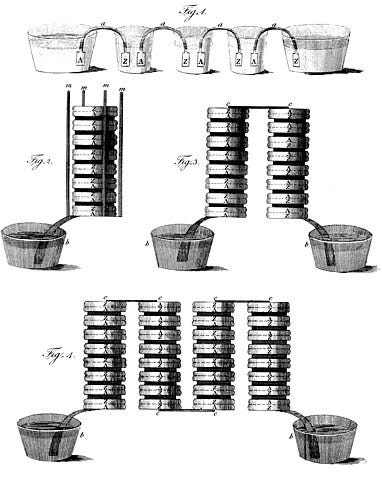
Look! These are the layers of discs! | Source: Wikipedia
Although studies of electricity had begun prior to Alessandro Volta’s groundbreaking invention in 1800 (from which we derive the term ‘volt’), his design offered a significant improvement as it did not require recharging—unlike devices like Leyden jars. This innovation paved the way for a new branch of chemistry, known as electrochemistry, which modern chemists often associate with battery technology. Ultimately, this development provided scientists with a new experimental tool.
Burning substances yields ash, a residue that has puzzled chemists since antiquity. Despite the extensive work of Antoine Lavoisier, even he could not decipher the nature of ash, suspecting it to be a compound but lacking definitive proof.
Enter Humphry Davy and his voltaic pile. Born in 1778 and raised on a farm, Davy initially worked as an assistant to a physician, only to be dismissed due to his penchant for “explosive experiments.” I can relate to that thrill of experimentation—had my parents been able to, they might have dismissed me too! Fortunately, Davy found another mentor who encouraged his scientific pursuits, allowing him to rise within the scientific community.
It's noteworthy that Davy's journey began from modest roots. While he may not be as widely recognized today as figures like John Dalton or Joseph Priestley, he exemplifies how determination and resilience can lead to significant contributions to science. Despite facing setbacks—such as being fired for his explosive experiments—Davy's adventurous spirit never waned, leading to pivotal discoveries that transformed chemistry.
Using one of the largest voltaic piles of his time, consisting of 100 discs, Davy experimented with “caustic potash,” or potassium hydroxide. He observed metallic droplets forming on his wire, which resembled mercury and exhibited spectacular explosions upon formation. Consequently, this element was named potassium, derived from “potash.” One can only imagine Davy's excitement upon making such a thrilling discovery.
Potassium is incredibly reactive and cannot be stored like most chemicals; it reacts violently with even trace amounts of moisture in the air. Thus, elemental potassium, which has a clay-like texture, is typically stored submerged in oil. The explosions associated with potassium are somewhat misleading; it is not potassium itself that detonates, but rather its reaction with water to produce hydrogen gas. This reaction generates a substantial amount of heat, resulting in explosive outcomes.
The first video showcases the explosive interaction between potassium and water, illustrating the dramatic effects of this reaction.
Shortly thereafter, Davy isolated sodium through similar experiments, which reacts in a less violent manner compared to potassium. He also discovered several other metals using this technique, including magnesium, calcium, strontium, and barium—all of which belong to the second column of the periodic table.
Davy's groundbreaking work attracted another notable scientist, Michael Faraday (1791–1867), who had also emerged from humble beginnings. Working in a bookbinding shop, Faraday taught himself chemistry during his teenage years. Unlike many of us, who were preoccupied with teenage distractions, Faraday exhibited the academic tenacity that often characterizes great minds. While most of Faraday's contributions were in physics, he did make one vital discovery in chemistry.

My aspirations as a teenager | Photo by Adam Whitlock on Unsplash
Faraday identified a direct correlation between the amount of electricity applied to a compound and the quantity of the resulting element. He even invented an instrument for measuring electrical current. This meant that a specific quantity of electricity would consistently yield a proportional amount of potassium from potash and sodium from soda ash. This revelation hinted at a connection between electricity and the atomic composition of elements, a concept that could have significantly advanced atomic theory had Faraday pursued it further. At that time, atomic theory was still in its infancy and lacked widespread acceptance.
Regardless, the era of electrical experimentation had begun. I like to humorously imagine Davy testing the electrodes of his voltaic piles on his tongue (a common childhood experiment with 9V batteries), which would align with his adventurous personality. One significant outcome from these electrical studies was the understanding that compounds exhibit electrical characteristics; that is, they are composed of positively and negatively charged ions. Faraday coined the terms “cations” and “anions” to describe these charged entities, a nomenclature that remains in use today. These investigations also laid the groundwork for a new theory of chemical bonding, known as the “dualistic” theory, which posited that compounds are made up of cations and anions. This theory aligned well with empirical data for many binary compounds, such as sodium chloride and potassium hydroxide, leading to its widespread acceptance.
While partially correct—since electrostatic attraction and repulsion play significant roles in chemical bonding—this theory oversimplified the complexities of chemical interactions. Nonetheless, it established new challenges for aspiring theorists to address.
As we delve deeper into the realm of chemistry, particularly organic chemistry, we will encounter various theoretical frameworks that have evolved over time. (I can almost hear a collective sigh from current and former pre-med students.) However, amidst the chaos of organic chemistry, there exists a bright spot—or perhaps a dark spot, depending on your experiences as a chemistry graduate student.
Chapter 2: The Impact of Electrochemistry on Chemistry
The second video highlights the properties and reactions of sodium, showcasing its fascinating yet less violent nature compared to potassium.
Works Consulted:
Brock, William H. The Chemical Tree: A History of Chemistry. New York: Norton and Co, 2000.
Ihde, Aaron J. The Development of Modern Chemistry. New York: Dover Publications, 1984.