Exploring the Epigenetics of Obesity: New Insights and Findings
Written on
Understanding Obesity
The prevalence of obesity is escalating globally, with certain Southern Pacific island nations reporting over 50% of their adult population classified as obese, followed closely by the United States at approximately 42%.
Obesity is clinically defined as a Body Mass Index (BMI) exceeding 30. However, BMI has its limitations; it does not account for variations in body composition. An individual with significant muscle mass may fall within the obese category despite being physically fit. A more precise definition considers the accumulation of excess body fat—generally, over 25% for men and over 30% for women. Nevertheless, BMI remains a useful tool for assessing population trends, as the majority of individuals are not excessively muscled.
Excess body fat serves various functions, including hormonal regulation, organ cushioning, energy provision, and aiding in vitamin absorption. However, carrying too much body fat significantly heightens the risk of numerous health issues, including heart disease, type 2 diabetes, sleep apnea, specific cancers, osteoarthritis, and asthma.
The accumulation of excess fat is fundamentally due to an energy imbalance; however, the situation is more intricate. Numerous factors—such as genetics, dietary surroundings, medical conditions, and medications—can influence this balance. While no one is fated to become obese, some individuals may naturally have a predisposition towards higher body fat percentages, necessitating more deliberate efforts to maintain a healthy weight.
The Role of Epigenetics in Obesity
Epigenetics involves the examination of molecular processes that regulate gene expression without modifying the underlying DNA sequence. This includes mechanisms such as DNA methylation and histone modifications. Epigenetics is vital during early development, determining the specific identity and function of cells, and it continues to play a significant role throughout life, including during aging.
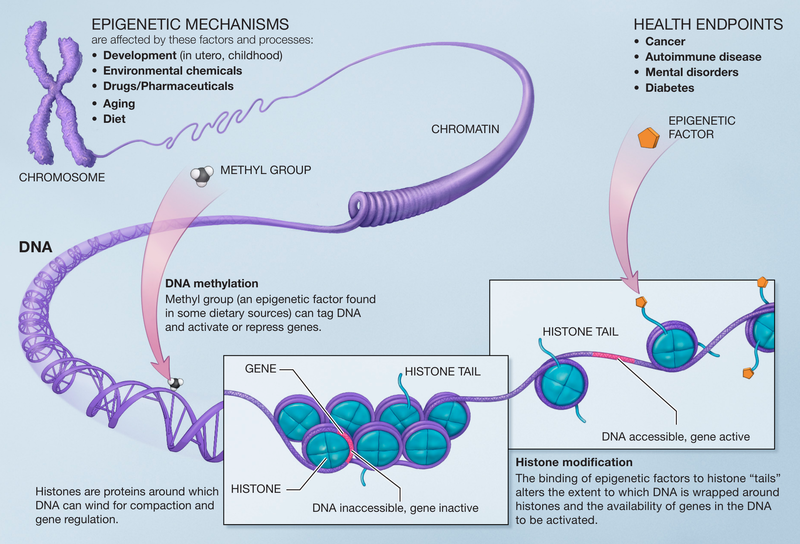
A crucial aspect of epigenetics is its capacity to allow environmental factors to influence genomic activity. Almost any environmental condition, from temperature to nutrition, can affect "epigenetic programming." Such programming may begin in utero, potentially altering the risk of obesity. Notably, children born to obese mothers are twice as likely to become obese themselves at a young age.
Conversely, a study noted that children born after their mothers underwent weight loss surgery exhibited nearly three times lower obesity rates compared to those born before this intervention. These children also showed improved insulin sensitivity, favorable lipid profiles, and lower levels of ghrelin (indicating reduced hunger) alongside elevated leptin (signifying easier satiety).
The researchers attributed these findings to the uterine environment and potential epigenetic impacts, though the exact mechanisms remain uncertain. A 2015 meta-analysis further supported this notion, identifying multiple obesity-related differentially methylated sites, particularly in blood cells, and observing minor changes in methylation during weight loss interventions.
Unraveling Insights through Twin Studies
Twins present an exceptional opportunity to control for genetic backgrounds in disease research. Studies have revealed that twins with identical genetic backgrounds can develop differing health conditions due to environmental influences acting through epigenetics.
A recent investigation focused on epigenetic "signatures" linked to obesity risk by analyzing twins with differing obesity statuses. This research involved both identical and fraternal twins from the Washington State Twin Registry, comprising 22 pairs (average age around 42 years, 55% female, predominantly white) who had significant BMI differences (ranging from 9 to 13 BMI points) and were free of certain medications and gastrointestinal conditions.
Using DNA samples obtained through buccal swabs, alongside body measurements and questionnaires, the researchers conducted a comprehensive analysis. They identified "differentially methylated regions" (DMRs) within the genome associated with genes and pathways pertinent to obesity. Through a method known as weighted genome co-expression network analysis (WGCNA), they identified gene networks related to specific population parameters, indicating changes in multiple genes' activities that suggest epigenetic "modules" influencing obesity.
The findings revealed that these epigenetic modules correlated with BMI and waist circumference in both identical and fraternal twins. In women, the modules linked to BMI and waist circumference were associated with soda and caffeine consumption, while in men, they correlated with the intake of energy drinks and fast food.
The researchers concluded that their observations demonstrate the potential for identifying systemic epigenetic biomarkers for obesity, which could pave the way for future clinical research involving larger sample sizes. The discovery of these biomarkers may enhance preventative strategies and early clinical interventions for obesity.
However, the authors correctly emphasized the need for further research with larger cohorts, as the current study's small scale limits the generalizability of the findings and complicates the determination of causality. Are these epigenetic signatures a precursor to obesity, or does being obese affect one's epigenetic markers? Likely, it is a combination of both.
This research represents an initial step toward uncovering potential epigenetic indicators of obesity risk, which is a significant advancement in the field.